PREVENTION OF SUDDEN CARDIAC DEATH AFTER MYOCARDIAL INFARCTION BY DEFIBRILLATOR IMPLANTATION
The PROFID EHRA trial is embedded in the large project PROFID, funded by the European Commission within the Horizon 2020 EU research and innovation programme, which is expected to change sudden cardiac death (SCD) prevention in clinical practice.

This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 847999.
ProFID EHRA TRIAL
Funded under: SOCIETAL CHALLENGES – Health, demographic change and well-being
Grant agreement ID: 847999
Chief Investigators: Gerhard Hindricks, MD / Nikolaos Dagres, MD
ClinicalTrials.gov Identifier: NCT05665608
STATUS profid EHRA trial
(as of 4 February 2026)
Participating clinical sites: 85*
(* 180 participating sites planned)
Enrolled patients: 292*
(* 3.595 enrolled patients planned)
Upcoming Events
28 November – 29 November 2025
Update on Sudden Cardiac Death 2025, Berlin/ DE
PROFID EHRA COLLABORATION
ESC TV 2024 – Interview
Background
current clinical guidelines
A severely reduced left ventricular ejection fraction (LVEF) after myocardial infarction (MI) was shown to indicate a higher risk for SCD and led to international guideline recommendations for routine implantation of defibrillators for primary prevention of SCD.

Current strategy for primary prevention of SCD*
Background and rationale of Profid
Only a minority of post-MI patients with LVEF ≤ 35% that currently receive a prophylactic implantable cardioverter defibrillator (ICD) will ever need the device – others are only exposed to potentially severe complications due to the implantation.
Existing data are outdated and do no longer represent current therapies, since SCD risk decreased during the last decades as a result of improved patient therapy.
Current publications have shown, that the risk-benefit of routine defibrillator implantation for primary prevention of SCD in patients with reduced LVEF has substantially changed during the last years, due to the change in the medical treatment of post-MI patients and constant complication rates associated with defibrillator therapy.
- A decreased benefit of prophylactic ICD implantation in heart failure patients due to novel drug classes that reduce mortality and SCD has been shown by Merchant, Levy & Kramer (2020). The full publication can be read here.

- A reduced SCD risk over the last two decades has been shown by Shen et al. (2017). The full publication can be read here.

A decreased annual shock rate over the last two decades has been shown by Sabbag et al. (2015).
- Substantial complication rates of ICD therapy exceeding 10% has been shown by van Barreveld et al. (2021). The full publication can be read here.

Thus, a novel randomised adequately powered assessment of the role of the defibrillator under contemporary optimal medical therapy (OMT) appears imperative
our aim in profid
The role of routine prophylactic ICD implantation in patients with reduced LVEF ≤ 35% after myocardial infarction under contemporary OMT for primary prevention of SCD and change medical guidelines needs to be reassessed.

“The PROFID EHRA trial will provide urgently needed contemporary data on sudden cardiac death prevention by defibrillator implantation after myocardial infarction and is expected to profoundly influence clinical practice.“
strategy
our strategy in profid
- Evaluate in a randomised controlled clinical trial the potential benefit or harm of routine prophylactic ICD implantation for primary prevention of SCD in the setting of contemporary OMT in post-MI patients with reduced LVEF ≤ 35%.
- Explore the potential of novel and promising risk markers for personalised risk prediction of SCD in two optional sub-studies (a cardiac Magnetic Resonance Imaging sub-study and a sub-study on genomics) and perform an artificial intelligence-based analysis of the twelve-lead Electrocardiograms (ECGs).
- Address ethical and legal aspects, including patients’ perspective on randomised therapy strategies for SCD prevention.
- Evaluate the economic impact to guide treatment decisions in patients with reduced LVEF ≤ 35%.
- Update corresponding European clinical guidelines on SCD prevention and ICD implantation.
profid EHRA trial
the profid EHRA trial (nct05665608)
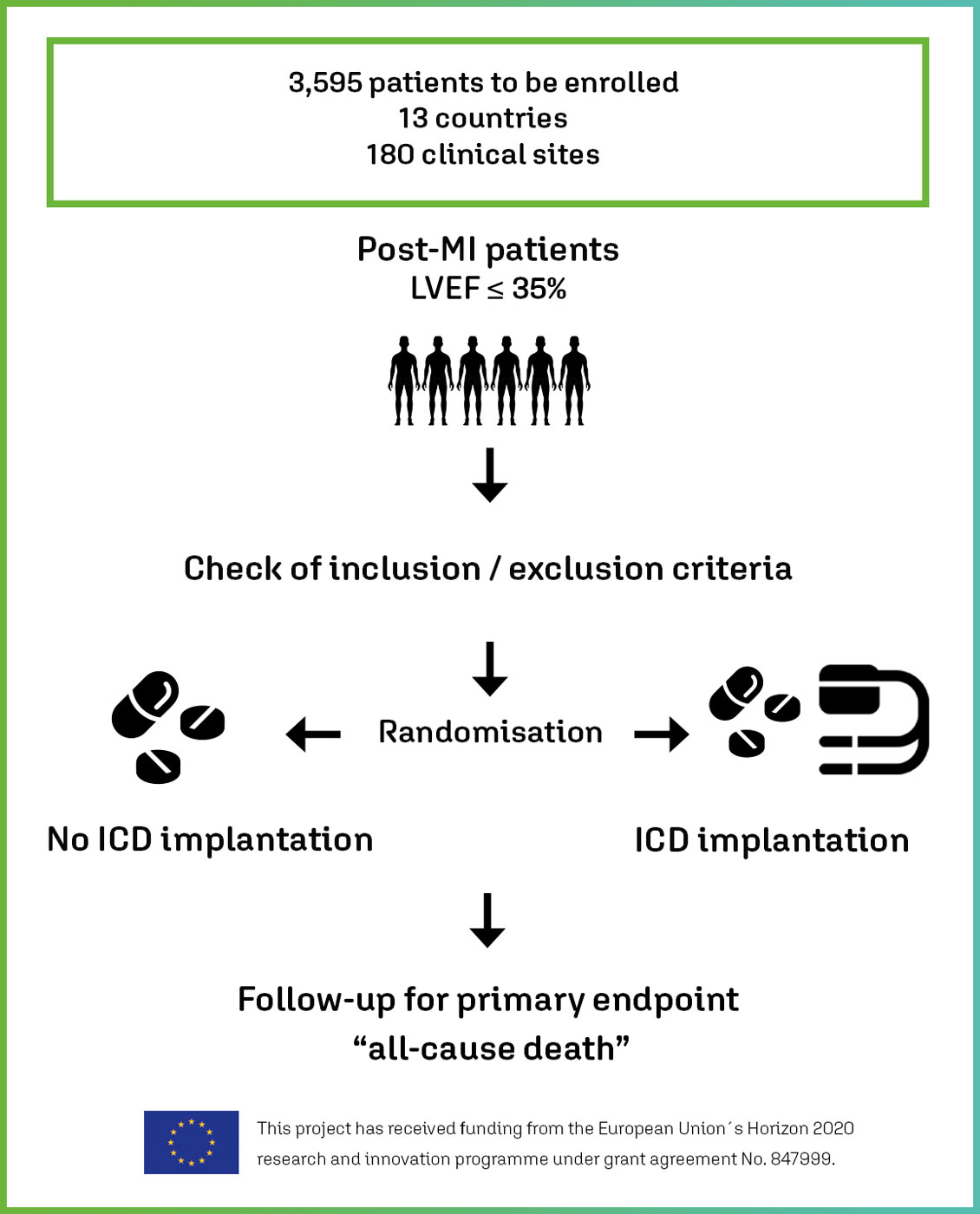
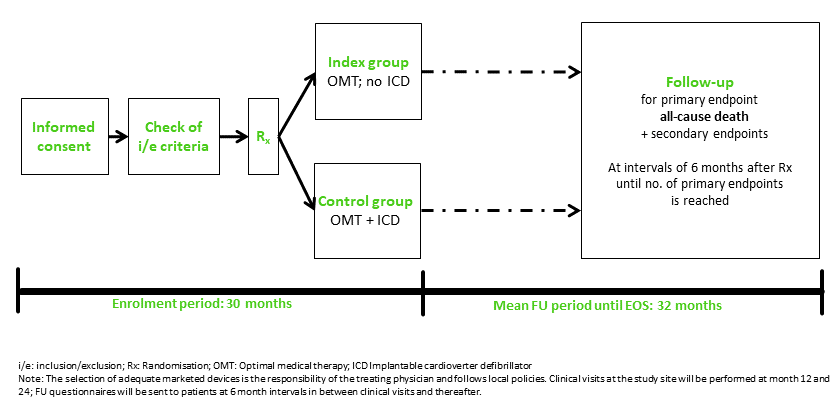
Non-inferiority, investigator-driven clinical trial with 3,595 randomised post-MI patients with symptomatic heart failure and reduced LVEF ≤ 35% who would receive an ICD according to current clinical guidelines.
Demonstrate that OMT without ICD implantation (index group) is not inferior to OMT with ICD implantation (control group) with respect to all-cause mortality within about 2.5 years of observation.
Designed to be as close to routine clinical care as possible to represent daily routine clinical care in an optimal way.
Publicly funded (EU Horizon 2020) and industry-independent without commercial interest or marketed products in focus.
Study design of the PROFID EHRA trial
Further information about the PROFID EHRA trial can be found at PROFID @ ClinicalTrials.gov.
Participating countries
Patient population
Key Inclusion criteria
- Naïve to implantation of any pacemaker or defibrillator.
- Documented history of MI either as STEMI or as NSTEMI at least 3 months prior to enrolment.
- Symptomatic heart failure with NYHA class II or III.
- On OMT for at least 3 months prior to enrolment.
- LVEF ≤35% (at TTE or cMRI at least 3 months after MI).
Key exclusion criteria
- Class I or IIa indication for an ICD implantation for secondary prevention of SCD and ventricular tachycardia.
- Ventricular tachycardia induced in an electrophysiologic study.
- Unexplained syncope when ventricular arrhythmia is suspected as the cause of syncope.
- Class I or IIa indication for Cardiac Resynchronization Therapy (CRT).
- Acute coronary syndrome or coronary angioplasty or CABG within 6 weeks prior to enrolment.
- Cardiac valve surgery or percutaneous cardiac valvular intervention within 6 weeks prior to enrolment.
- On the waiting list for heart transplantation.
Study flow chart
contact profid EHRA trial
clinical research organization
CRI – The Clinical Research Institute GmbH – Now part of NAMSA
Rosa-Bavarese-Str. 3
80639 Munich / Germany
Profid Hotline: +49 89 990 1649 974
sponsor
Charité – Universitätsmedizin Berlin
Charitéplatz 1
10117 Berlin / Germany